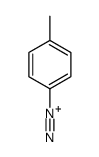
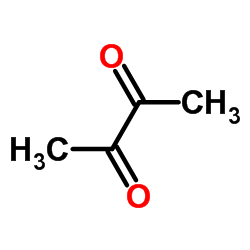
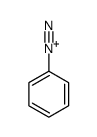
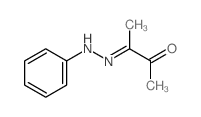
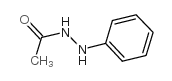
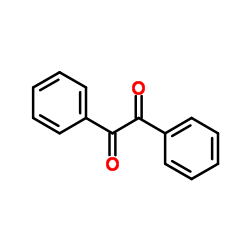
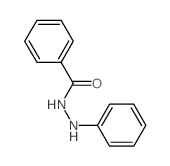
|
~67% |
|
~16% |
|
~5%
Detail
|