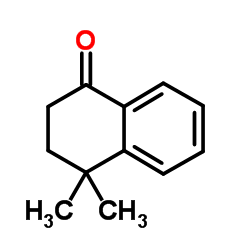
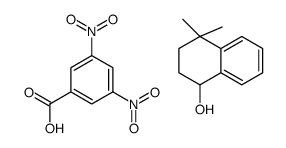
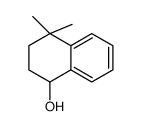
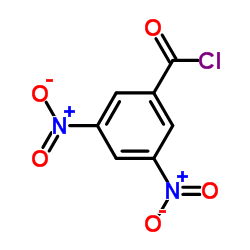
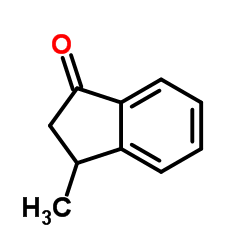
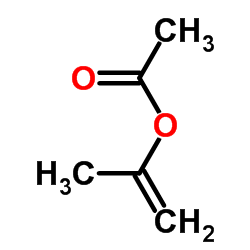
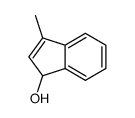
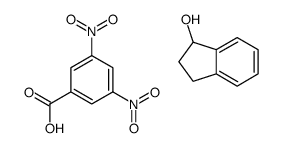
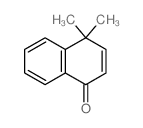
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
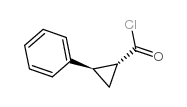
![1a,6a-dihydro-1H-cyclopropa[a]inden-6-one Structure](https://image.chemsrc.com/caspic/340/5771-62-0.png)