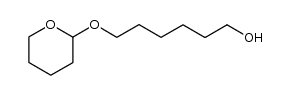
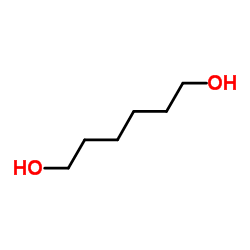
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~% |
|
~% |
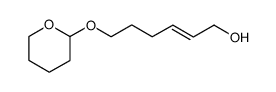


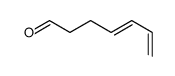
![(E)-9-[(tetrahydro-2H-pyran-2-yl)oxy]-1,3-nonadiene Structure](https://image.chemsrc.com/caspic/269/167419-06-9.png)
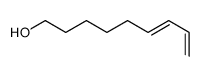
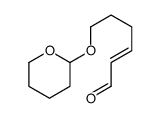
![6-[(3,4,5,6-tetrahydro-2H-pyran-2-yl)oxy]hexanal Structure](https://image.chemsrc.com/caspic/271/33803-62-2.png)