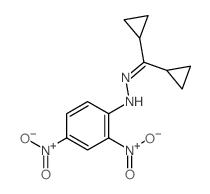
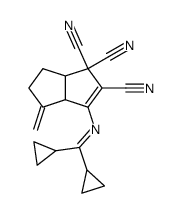
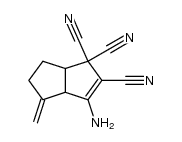
|
~29% |
|
~28% |
|
~0% |
|
~0% |
![Spiro[2.4]hepta-4,6-diene Structure](https://image.chemsrc.com/caspic/060/765-46-8.png)
![spiro[2.4]hept-6-ene Structure](https://image.chemsrc.com/caspic/442/52708-23-3.png)
![spiro[2.4]heptane Structure](https://image.chemsrc.com/caspic/388/185-49-9.png)
![1-((dicyclopropylmethylene)amino)-8-methylene-8,8a-dihydrocyclopenta[a]indene-2,3,3(3aH)-tricarbonitrile Structure](https://image.chemsrc.com/caspic/443/142422-03-5.png)