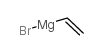
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~34% |
|
~% |
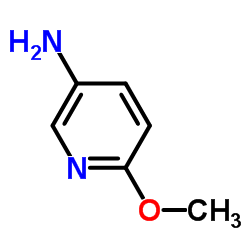
![4-Fluoro-1H-pyrrolo[2,3-c]pyridine-7-carbonitrile Structure](https://image.chemsrc.com/caspic/187/446284-50-0.png)
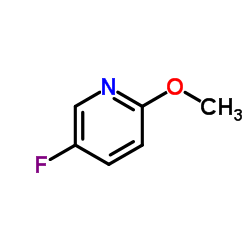
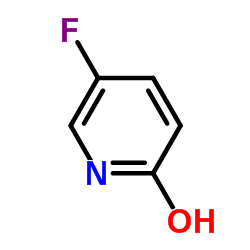
![4-Fluoro-1H-pyrrolo[2,3-c]pyridine-7-carboxylic acid Structure](https://image.chemsrc.com/caspic/459/446284-56-6.png)
![7-Bromo-4-fluoro-1H-pyrrolo[2,3-c]pyridine Structure](https://image.chemsrc.com/caspic/297/446284-38-4.png)

![4-Fluoro-1H-pyrrolo[2,3-c]pyridine-7-carbaldehyde Structure](https://image.chemsrc.com/caspic/166/446284-46-4.png)