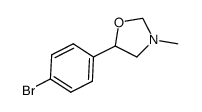
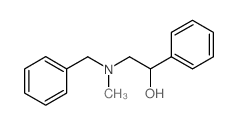
|
~% |
|
~% |
|
~% |
|
~% |

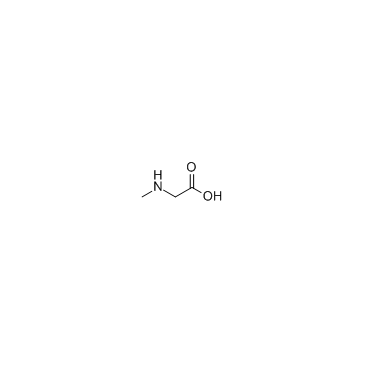


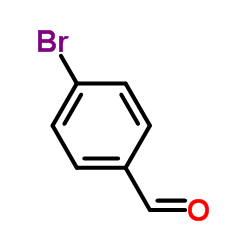
![4-(4-bromophenyl)-6-methyl-5,7-dihydro-4H-thieno[2,3-c]pyridine Structure](https://image.chemsrc.com/caspic/172/70696-52-5.png)