|
~37% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~47% |
|
~% |
|
~% |
|
~% |
![[3-(6-amino-purin-7-yl)-4-trityloxy-butoxymethyl]phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/415/918795-52-5.png)
![[3-(6-aminopurin-7-yl)-4-hydroxybutoxy]methylphosphonic acid Structure](https://image.chemsrc.com/caspic/387/848782-36-5.png)

![[3,4-dihydroxybutoxymethyl]-phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/215/918795-57-0.png)
![[2-(2,2-dimethyl[1,3]dioxolan-4-yl)ethoxymethyl]-phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/263/918795-56-9.png)
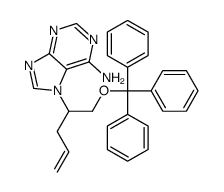
![[3-hydroxy-4-trityloxy-butoxymethyl]-phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/284/918795-60-5.png)


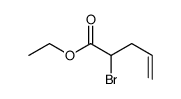
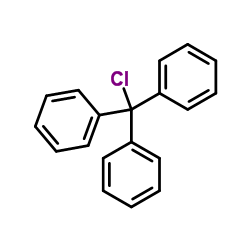
![[2-(6-amino-purin-7-yl)-4-hydroxy-butoxymethyl]-phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/432/918795-47-8.png)
![[2-(6-aminopurin-7-yl)-4-hydroxybutoxy]methylphosphonic acid Structure](https://image.chemsrc.com/caspic/311/848782-38-7.png)
![[2-(6-amino-purin-7-yl)-pent-4-enyloxymethyl]-phosphonic acid diisopropyl ester Structure](https://image.chemsrc.com/caspic/122/918795-42-3.png)
