|
~24% |
|
~% |
|
~% |
|
~% |
![Benzene,[(1-methylethyl)sulfinyl] Structure](https://image.chemsrc.com/caspic/469/4170-69-8.png)
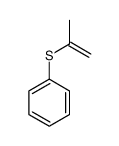


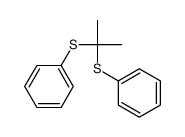
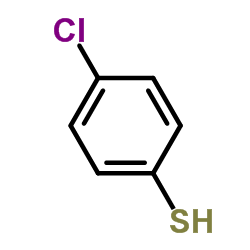
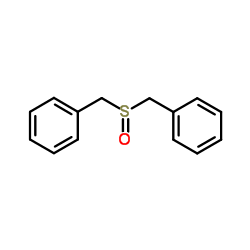
![[benzylsulfanyl(phenyl)methyl]sulfanylbenzene Structure](https://image.chemsrc.com/caspic/373/62740-57-2.png)