|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
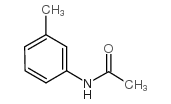
![3-[2-(4-amino-3-methylphenyl)ethylamino]propane-1-sulfonic acid Structure](https://image.chemsrc.com/caspic/311/75489-04-2.png)
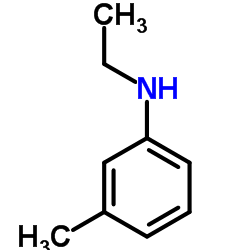
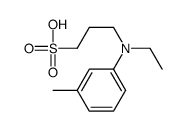
![N,N-Bis-[β-hydroxyethyl]-3-methyl-4-nitroso-anilin Structure](https://image.chemsrc.com/caspic/434/103640-03-5.png)
![2-[4-amino-N-(2-hydroxyethyl)-3-methylanilino]ethanol Structure](https://image.chemsrc.com/caspic/246/2359-52-6.png)

