|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![4,8,8-trichloro-2,3,5-trimethyl-bicyclo[4.2.0]octa-2,4,9-triene Structure](https://image.chemsrc.com/caspic/146/6590-37-0.png)
![ethyl 5-[[3-(4-methoxyphenyl)-1-phenylpyrazole-4-carbonyl]amino]-3-methylthiophene-2-carboxylate Structure](https://image.chemsrc.com/caspic/215/6590-40-5.png)

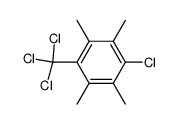
![2,3,4,5-Tetramethyl-bicyclo[4.2.0]octa-1,3,5-trien-7-ol Structure](https://image.chemsrc.com/caspic/050/6670-39-9.png)
![2,3,4,5-tetramethylbicyclo[4.2.0]octa-1,3,5-triene-8-carbonitrile Structure](https://image.chemsrc.com/caspic/088/6670-38-8.png)
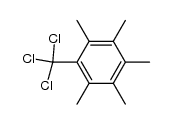
![7,7-dichloro-2,3,4,5-tetramethylbicyclo[4.2.0]octa-1,3,5-triene Structure](https://image.chemsrc.com/caspic/148/6663-26-9.png)
![2,3,4,5-tetramethylbicyclo[4.2.0]octa-1,3,5-trien-7-one Structure](https://image.chemsrc.com/caspic/194/6590-36-9.png)
![7-Brom-2,3,4,5-tetramethyl-bicyclo[4,2,0]octa-1,3,5-trien Structure](https://image.chemsrc.com/caspic/257/6670-29-7.png)
