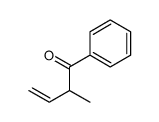
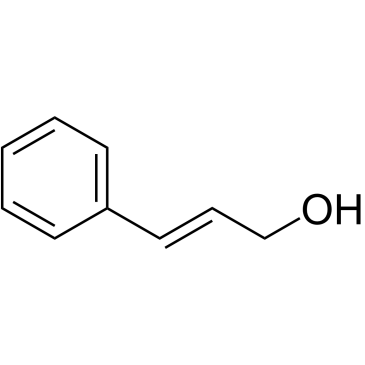
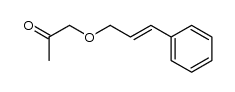
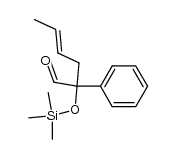
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

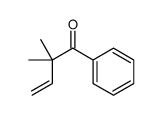

![[(1,1-dimethylallyl)oxy]acetophenone Structure](https://image.chemsrc.com/caspic/244/65755-83-1.png)

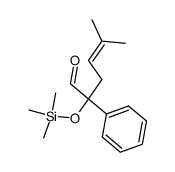
![2-[(1,1-dimethylallyl)oxy]-1-phenylethanol Structure](https://image.chemsrc.com/caspic/137/100702-20-3.png)