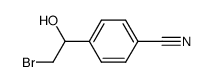
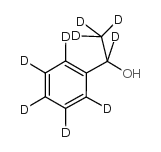
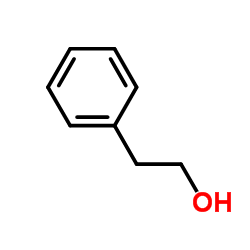
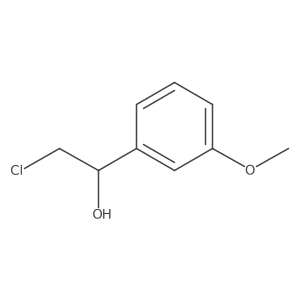
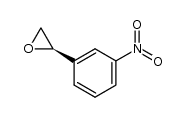
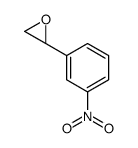
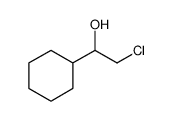
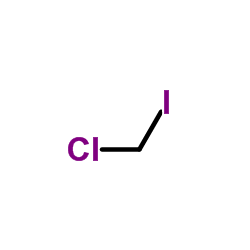
|
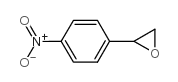
~59% |
|
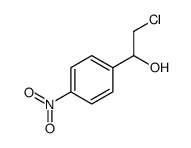
~% |
|
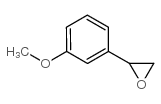
~0% |
|
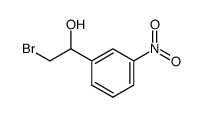
~57% |
|
~71% |
|
~% |
|
~% |
|
~% |
|
~% |
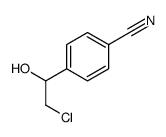
![4-[(2R)-oxiran-2-yl]benzonitrile Structure](https://image.chemsrc.com/caspic/060/179694-34-9.png)