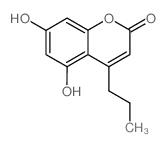
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

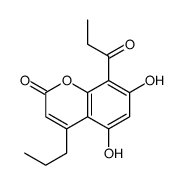
![5-hydroxy-2,2-dimethyl-10-propyl-6-propionyl-2H,8H-pyrano[2,3-f]chromen-8-one Structure](https://image.chemsrc.com/caspic/021/166983-61-5.png)
![2H,6H,10H-Dipyrano[2,3-f:2',3'-h][1]benzopyran-2-one,11,12-dihydro-12-hydroxy-6,6,10,11-tetramethyl-4-propyl-, (10R,11S,12S) Structure](https://image.chemsrc.com/caspic/409/142632-32-4.png)