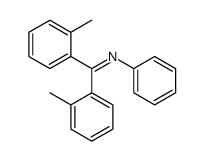
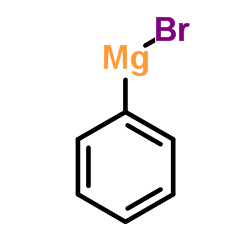
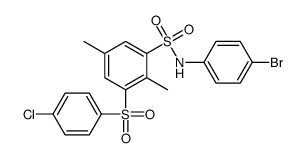
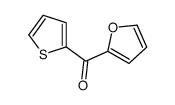
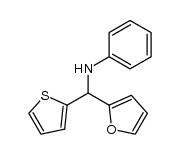
|
~92% |
|
~% |
|
~92% |
|
~99% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~89% |
|
~83% |
|
~% |
|
~10% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
![N-[di(2-thiazolyl)methylene]aniline Structure](https://image.chemsrc.com/caspic/167/123209-19-8.png)
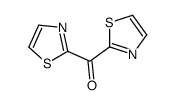

![N-[α-(2-furanyl)benzylidene]aniline Structure](https://image.chemsrc.com/caspic/264/123209-21-2.png)
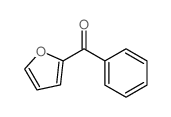

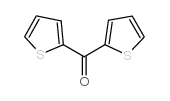
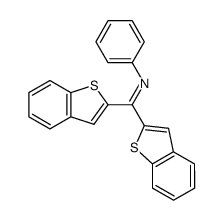
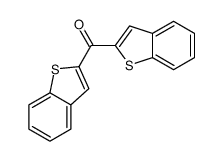
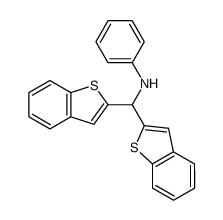
![N-[α-(2-furanyl)benzyl]aniline Structure](https://image.chemsrc.com/caspic/249/36749-19-6.png)
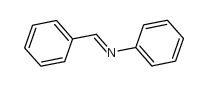
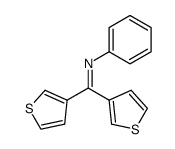
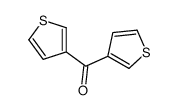

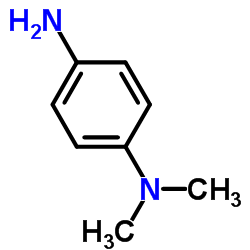
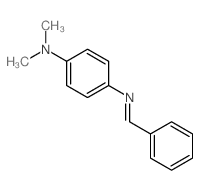
![N-[bis(1-methylpyrrol-2-yl)methylene]aniline Structure](https://image.chemsrc.com/caspic/302/123209-20-1.png)
![N-[bis(1-methylpyrrol-2-yl)methyl]aniline Structure](https://image.chemsrc.com/caspic/395/123209-13-2.png)