|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~71% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
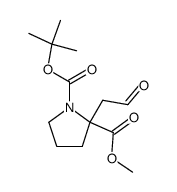
![2-Methyl-2-propanyl (5R)-6-oxo-7-[(1S)-1-phenylethyl]-1,7-diazasp iro[4.4]nonane-1-carboxylate Structure](https://image.chemsrc.com/caspic/318/908264-55-1.png)
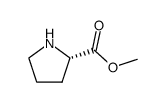

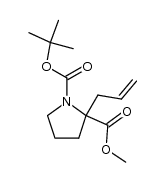
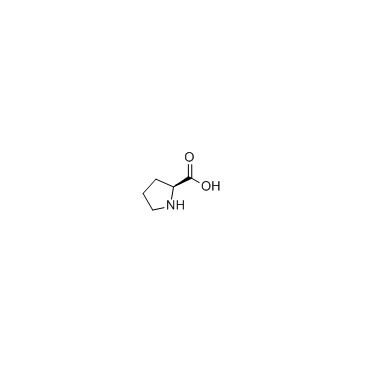
![2-Methyl-2-propanyl (5S)-6-oxo-7-[(1R)-1-phenylethyl]-1,7-diazasp iro[4.4]nonane-1-carboxylate Structure](https://image.chemsrc.com/caspic/187/908264-63-1.png)
![2-Methyl-2-propanyl (5S)-6-oxo-7-[(1S)-1-phenylethyl]-1,7-diazasp iro[4.4]nonane-1-carboxylate Structure](https://image.chemsrc.com/caspic/111/908264-65-3.png)
![2-Methyl-2-propanyl (5R)-6-oxo-7-[(1R)-1-phenylethyl]-1,7-diazasp iro[4.4]nonane-1-carboxylate Structure](https://image.chemsrc.com/caspic/442/908264-52-8.png)
![(5S)-7-[(1R)-1-Phenylethyl]-1,7-diazaspiro[4.4]nonan-6-one Structure](https://image.chemsrc.com/caspic/099/951167-15-0.png)
![(5R)-7-[(1S)-1-Phenylethyl]-1,7-diazaspiro[4.4]nonan-6-one Structure](https://image.chemsrc.com/caspic/061/951167-16-1.png)
![(5S)-7-[(1S)-1-Phenylethyl]-1,7-diazaspiro[4.4]nonan-6-one Structure](https://image.chemsrc.com/caspic/023/951167-17-2.png)