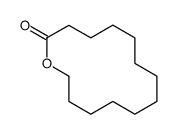
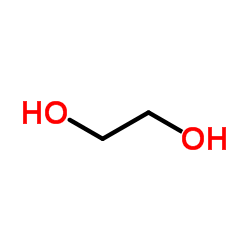
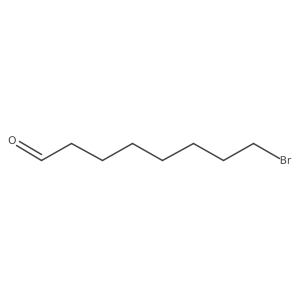
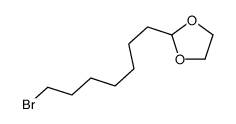
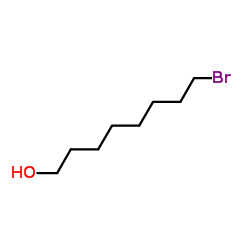
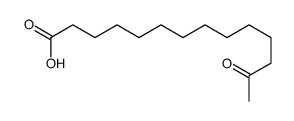
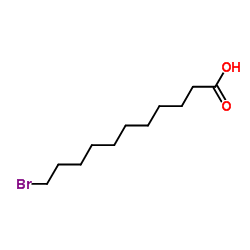
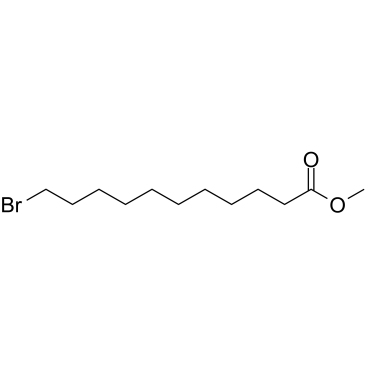
|
~96% |
|
~% |
|
~98% |
|
~93% |
|
~% |
|
~89% |
|
~% |
|
~% |

![2-[4-(1,3-dithian-2-yl)butoxy]oxane Structure](https://image.chemsrc.com/caspic/182/111741-88-9.png)