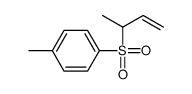
|
~% |
|
~% |
|
~% |
|
~% |
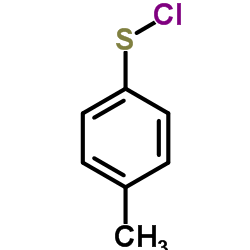
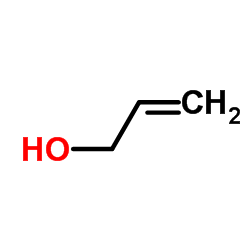

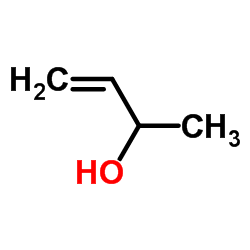
![1-[(E)-but-2-enyl]sulfonyl-4-methylbenzene Structure](https://image.chemsrc.com/caspic/441/24931-66-6.png)