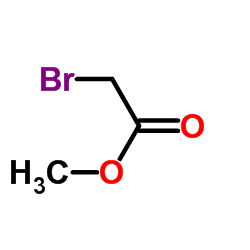
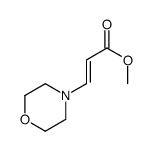
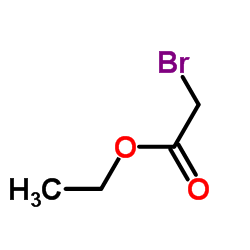
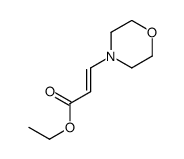
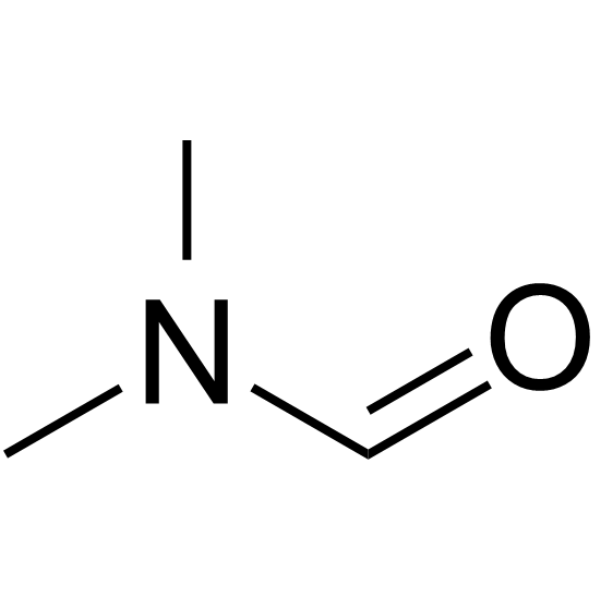
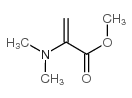
|
~64% |
|
~61% |
|
~65% |
|
~66% |