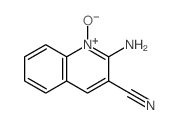
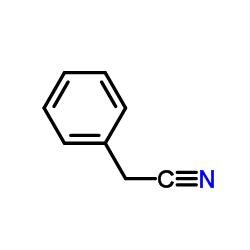
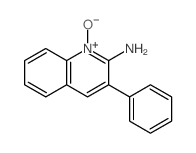
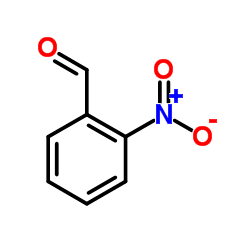
|
~81% |
|
~% |
|
~% |
|
~88% |
|
~67% |
|
~% |
|
~% |

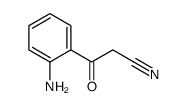
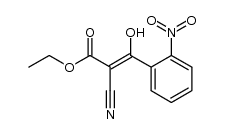
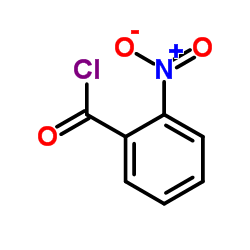
![Propanedinitrile,2-[(2-nitrophenyl)methylene] Structure](https://image.chemsrc.com/caspic/123/2826-30-4.png)