|
~72% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~0% |
|
~% |
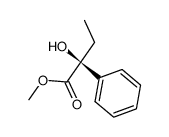
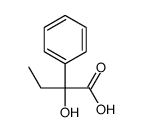
![phenyl((2R,4aR,7R,8aR)-4,4,7-trimethylhexahydro-4H-benzo[e][1,3]oxathiin-2-yl)methanone Structure](https://image.chemsrc.com/caspic/264/266694-57-9.png)
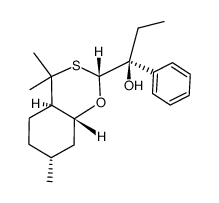

![phenyl((2S,4aR,7R,8aS)-4,4,7-trimethylhexahydro-4H-benzo[e][1,3]oxathiin-2-yl)methanone Structure](https://image.chemsrc.com/caspic/379/89616-56-8.png)

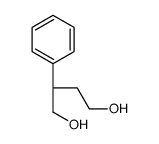
![(3aR,6R,7aR)-3,3,6-trimethylhexahydro-3H-benzo[d][1,2]oxathiole 2-oxide Structure](https://image.chemsrc.com/caspic/111/79563-63-6.png)


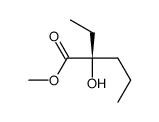
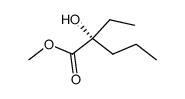
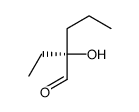
![3-((2S,4aR,7R,8aS)-4,4,7-trimethylhexahydro-4H-benzo[e][1,3]oxathiin-2-yl)hexan-3-ol Structure](https://image.chemsrc.com/caspic/417/89616-55-7.png)