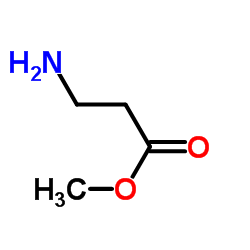
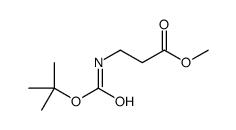
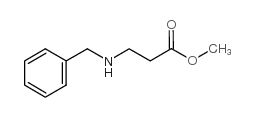
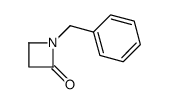
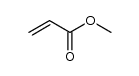
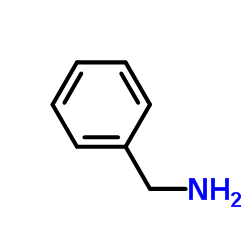
|
~99% |
|
~20% |
|
~97% |
|
~98% |