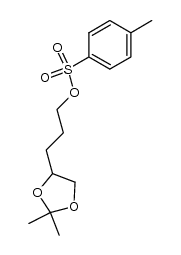
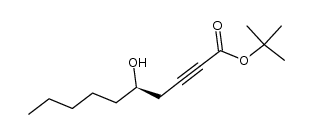
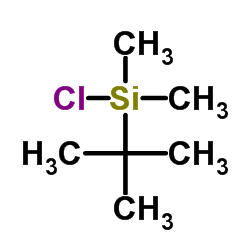
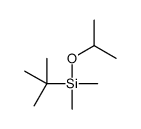
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~77% |
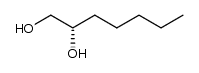

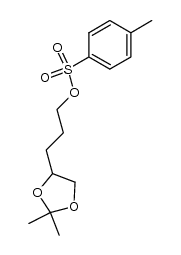
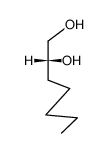

![2,2-dimethyl-4-pentyl-[1,3]dioxolane Structure](https://image.chemsrc.com/caspic/299/60045-13-8.png)