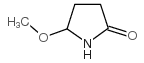
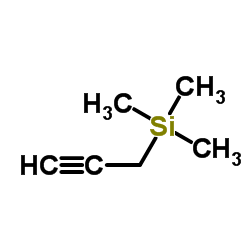
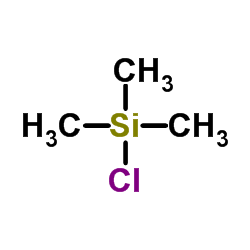
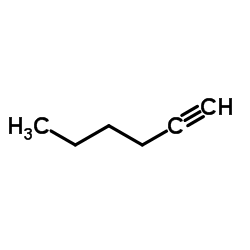
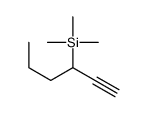
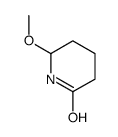
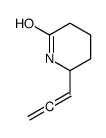
|
~45% |
|
~57% |
|
~72% |
|
~54% |