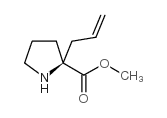
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
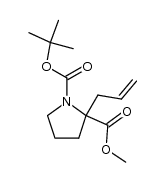
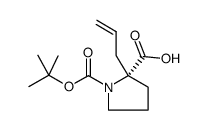
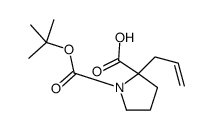
![2-Methyl-2-propanyl (5S)-8-hydroxy-6-oxo-7-oxa-1-azaspiro[4.4]non ane-1-carboxylate Structure](https://image.chemsrc.com/caspic/489/797760-59-9.png)

![2-Methyl-2-propanyl (5R)-8-hydroxy-6-oxo-7-oxa-1-azaspiro[4.4]non ane-1-carboxylate Structure](https://image.chemsrc.com/caspic/336/731807-68-4.png)