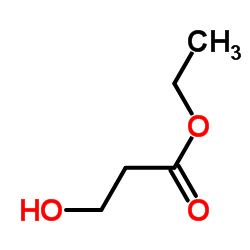
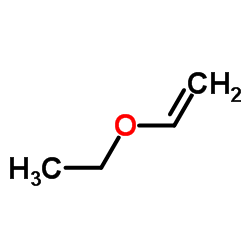
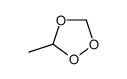
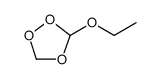
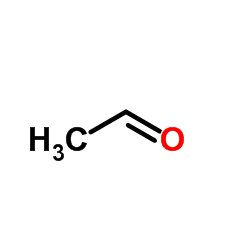
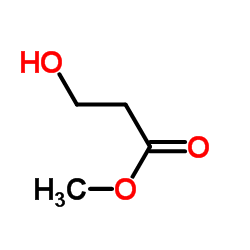
|
~64% |
|
~15% |
|
~14%
Detail
|
|
~51% |