|
~% |
|
~81% |
|
~% |
|
~93% |

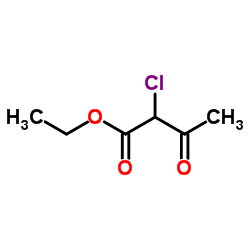
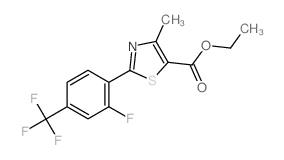
![4-[[[2-[2-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-5-thiazolyl]methyl]thio]-2-methylphenol Structure](https://image.chemsrc.com/caspic/044/447406-50-0.png)
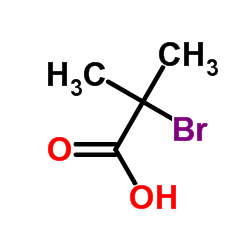
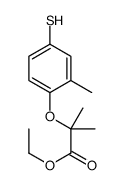
![2-[4-[[2-[2-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methylsulfanyl]-2-methylphenoxy]-2-methylpropanoic acid Structure](https://image.chemsrc.com/caspic/316/447406-78-2.png)
![[2-[2-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methanol Structure](https://image.chemsrc.com/caspic/402/317319-36-1.png)