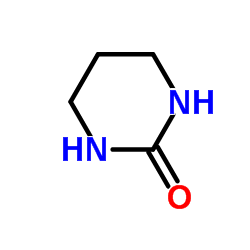
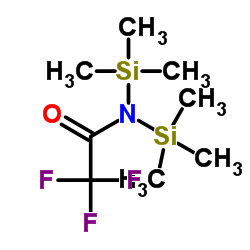
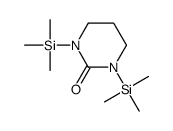
|
~% |
|
~85% |
|
~% |
|
~83% |
|
~% |
|
~% |
|
~% |
|
~% |
![2-[4-(1,3-dioxoisoindol-2-yl)but-2-enyl]isoindole-1,3-dione Structure](https://image.chemsrc.com/caspic/046/40794-71-6.png)

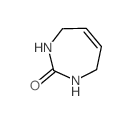
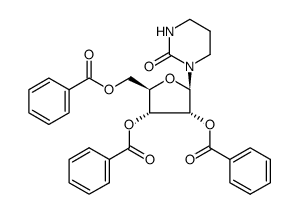
![1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-diazinan-2-one Structure](https://image.chemsrc.com/caspic/159/19149-48-5.png)
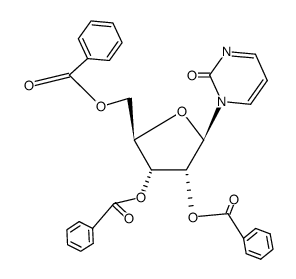

![1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-diazepan-2-one Structure](https://image.chemsrc.com/caspic/352/74024-66-1.png)
![1,3-bis-trimethylsilanyl-[1,3]diazepan-2-one Structure](https://image.chemsrc.com/caspic/084/18023-41-1.png)