|
~% |
|
~% |
|
~% |
|
~78% |
|
~% |
|
~% |

![Coumarin, 7-[ (6,7-dihydroxy-3,7-dimethyl-2-octenyl)oxy] Structure](https://image.chemsrc.com/caspic/231/5980-09-6.png)
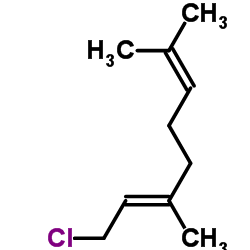
![(E)-7-[[5-(3,3-dimethyloxiranyl)-3-methyl-2-pentenyl]oxy]-2H-1-benzopyran-2-one Structure](https://image.chemsrc.com/caspic/299/36414-00-3.png)
![(S)-7-[5-(3,3-dimethyloxiranyl)-3-methylpent-2E-enyloxy]chromen-2-one Structure](https://image.chemsrc.com/caspic/230/118628-99-2.png)

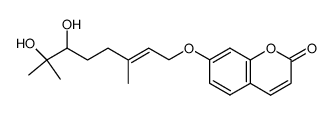
![7-[(E)-7-hydroxy-3,7-dimethyl-6-oxo-oct-2-enoxy]chromen-2-one Structure](https://image.chemsrc.com/caspic/034/36413-96-4.png)