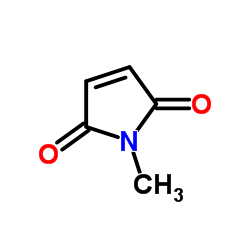
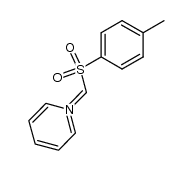
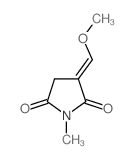
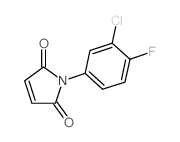
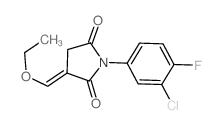
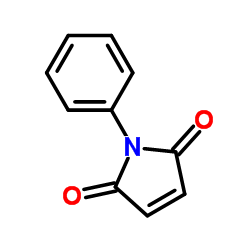
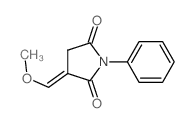
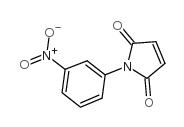
|
~77% |
|
~34% |
|
~62% |
|
~65% |