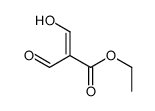
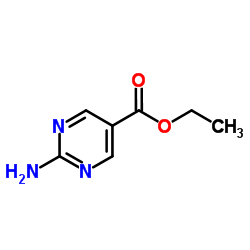
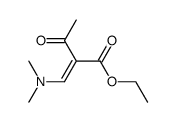
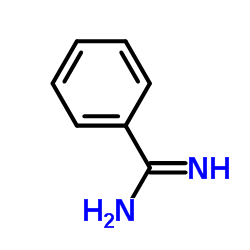
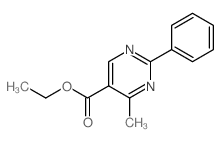
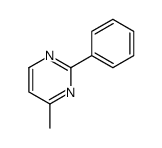
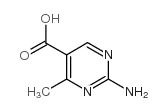
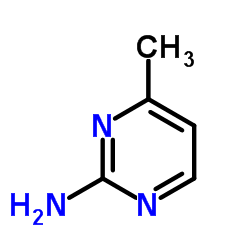
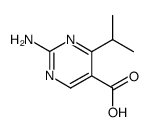
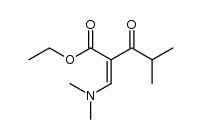
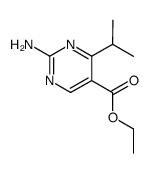
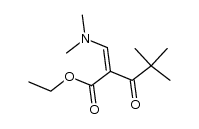
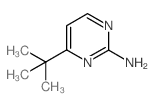
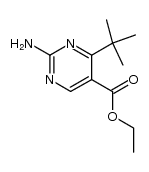
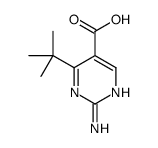
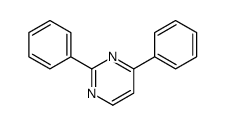
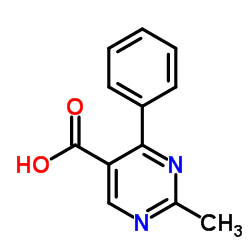
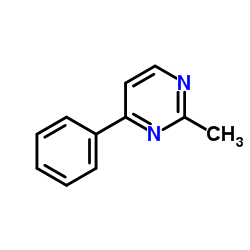
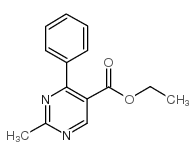
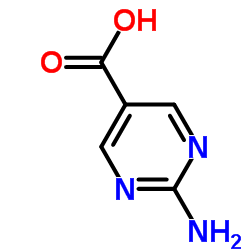
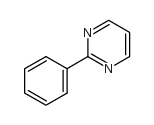
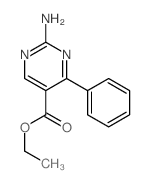
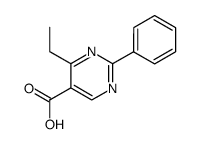
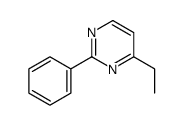
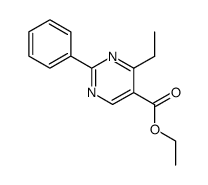
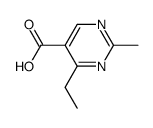
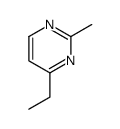
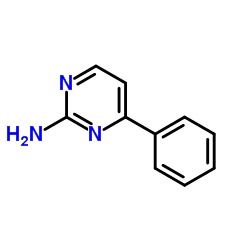
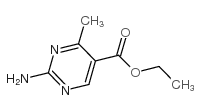
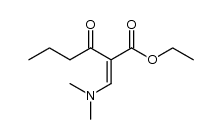
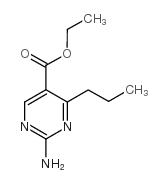
|
~34% |
|
~65% |
|
~94% |
|
~% |
|
~85% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~73% |
|
~89% |
|
~77% |
|
~88% |
|
~% |
|
~% |
|
~82% |
|
~51% |
|
~93% |
|
~% |
|
~38% |
|
~93% |
|
~% |
|
~% |
|
~69% |
|
~73% |
|
~70% |
|
~82% |