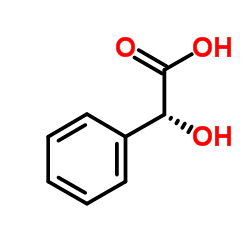
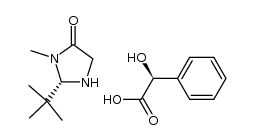
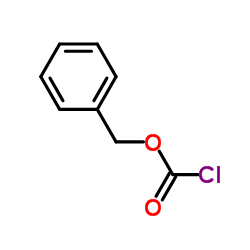
|
~30% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~43% |
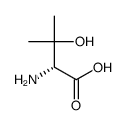
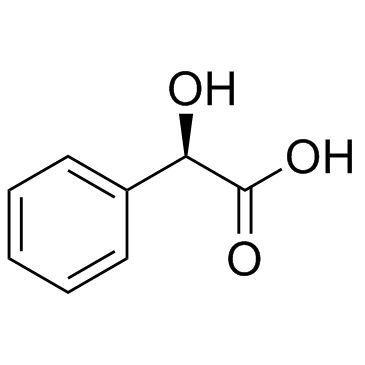



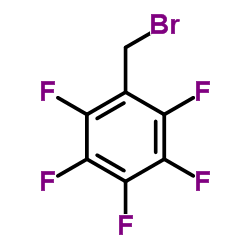
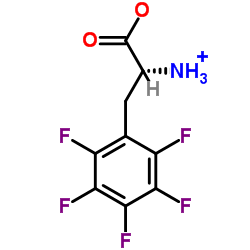

![(2-P-TOLYL-IMIDAZO[1,2-A]PYRIDIN-3-YL)-ACETONITRILE Structure](https://image.chemsrc.com/caspic/178/101055-57-6.png)