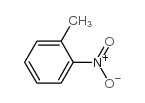
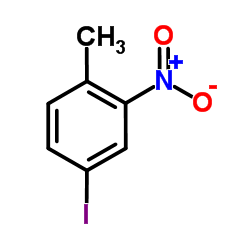
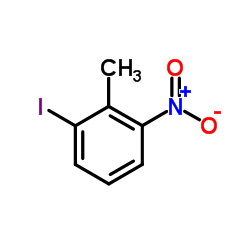
|
~77% |
|
~65% |
|
~23% |
|
~22% |