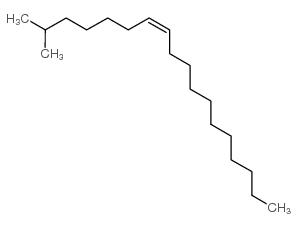
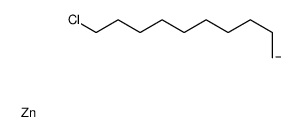
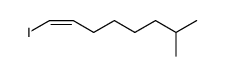
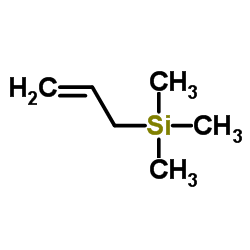
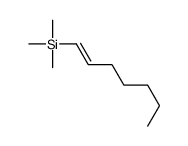
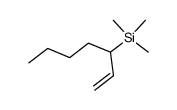
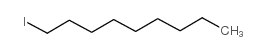
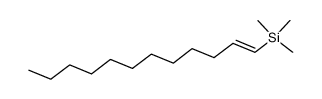
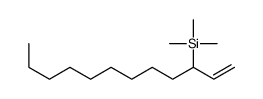
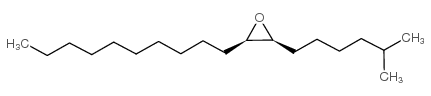
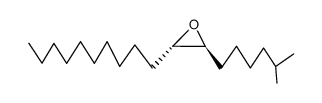
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~0% |
|
~13% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

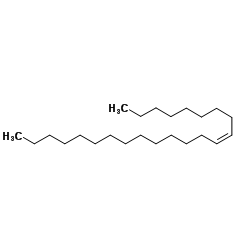
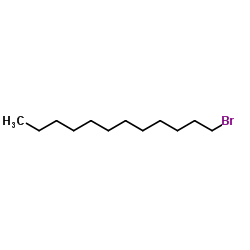
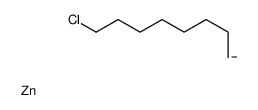

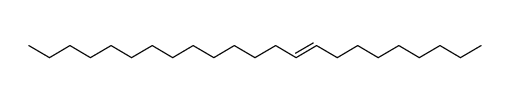
![Silane, trimethyl[(1E)-7-methyl-1-octen-1-yl] Structure](https://image.chemsrc.com/caspic/073/90359-67-4.png)