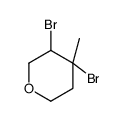
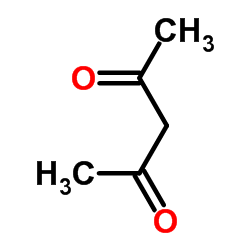
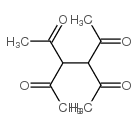
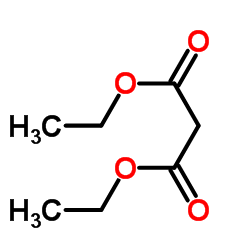
Efficient Selective Formation of C??C Single Bonds and C˭ C ...
[Wang, Zhiguo; Yin, Guodong; Chen, Aihua; Hu, Shengli; Wu, Anxin Synthetic Communications, 2007 , vol. 37, # 24 p. 4399 - 4405]
|
One-step preparation of symmetrical 1, 4-diketones from α-ha...
[Ceylan, Mustafa; Guerdere, M. Burcu; Budak, Yakup; Kazaz, Cavit; Secen, Hasan Synthesis, 2004 , # 11 p. 1750 - 1754]
|
Electrosynthesis of organic compounds. XII: Synthesis of som...
[Ismail, M. T. Bulletin de la Societe Chimique de France, 1987 , # 3 p. 438 - 440]
|
Electrosynthesis of organic compounds. XII: Synthesis of som...
[Ismail, M. T. Bulletin de la Societe Chimique de France, 1987 , # 3 p. 438 - 440]
|
Electrosynthesis of organic compounds. XII: Synthesis of som...
[Ismail, M. T. Bulletin de la Societe Chimique de France, 1987 , # 3 p. 438 - 440]
|