|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
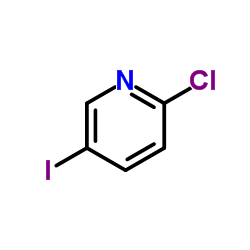
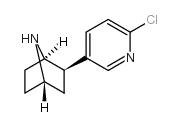

![(1S,2R,5S)-5-methyl-2-(2-(naphthalen-2-yl)propan-2-yl)cyclohexyl (1S,4R)-5-(6-chloropyridin-3-yl)-2-oxa-3-azabicyclo[2.2.2]oct-5-ene-3-carboxylate Structure](https://image.chemsrc.com/caspic/359/211503-87-6.png)
![(1S,4R,5R)-tert-butyl 5-(6-chloropyridin-3-yl)-2-oxa-3-azabicyclo[2.2.2]octane-3-carboxylate Structure](https://image.chemsrc.com/caspic/435/211503-85-4.png)
![(1S,2R,5S)-5-methyl-2-(2-(naphthalen-2-yl)propan-2-yl)cyclohexyl (1S,4R,5R)-5-(6-chloropyridin-3-yl)-2-oxa-3-azabicyclo[2.2.2]octane-3-carboxylate Structure](https://image.chemsrc.com/caspic/418/211503-90-1.png)