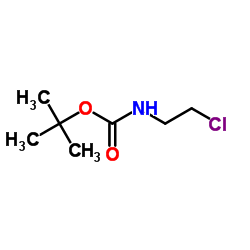
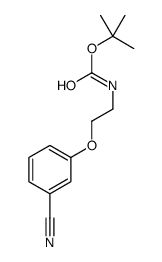
|
~91% |
|
~97% |
|
~77% |
|
~% |
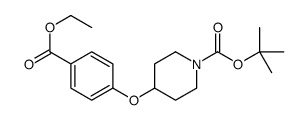
![4-{[1-(tert-Butoxycarbonyl)piperidin-4-yl]oxy}benzoic acid Structure](https://image.chemsrc.com/caspic/190/162046-56-2.png)