|
~76% |
|
~76% |
|
~76% |
|
~76% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~94% |
![(3aR,6S,6aR)-2,2-dimethyl-6-((2-phenylpropan-2-yl)oxy)dihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one Structure](https://image.chemsrc.com/caspic/132/288269-07-8.png)
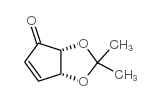
![(3aR,6S,6aS)-6-((tert-butyldimethylsilyl)oxy)-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one Structure](https://image.chemsrc.com/caspic/169/273381-26-3.png)
![(3aS,6R,6aS)-2,2-dimethyl-6-((2-phenylpropan-2-yl)oxy)dihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one Structure](https://image.chemsrc.com/caspic/283/273381-23-0.png)
![({5-[(3-METHOXYPHENYL)AMINO]-1,3,4-THIADIAZOL-2-YL}THIO)ACETICACID Structure](https://image.chemsrc.com/caspic/175/104010-72-2.png)
![(3aS,6R,6aR)-6-((tert-butyldimethylsilyl)oxy)-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one Structure](https://image.chemsrc.com/caspic/208/288269-05-6.png)
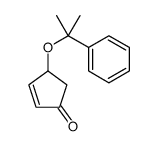
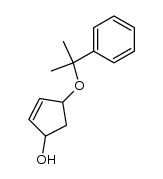

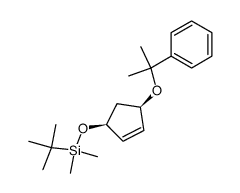

![tert-butyl(((3aR,4R,6S,6aR)-2,2-dimethyl-6-((2-phenylpropan-2-yl)oxy)tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl)oxy)dimethylsilane Structure](https://image.chemsrc.com/caspic/246/288269-04-5.png)
![(3aR,4S,6R,6aS)-2,2-dimethyl-6-((2-phenylpropan-2-yl)oxy)tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol Structure](https://image.chemsrc.com/caspic/331/273381-22-9.png)
![(3aR,4S,6R,6aR)-6-((tert-butyldimethylsilyl)oxy)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol Structure](https://image.chemsrc.com/caspic/110/273750-68-8.png)
![tert-butyl(((3aS,4S,6R,6aS)-2,2-dimethyl-6-((2-phenylpropan-2-yl)oxy)tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl)oxy)dimethylsilane Structure](https://image.chemsrc.com/caspic/369/273381-21-8.png)