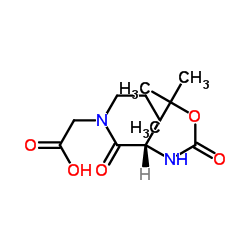
|
~80% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~99% |
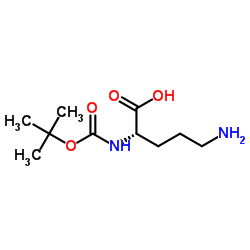
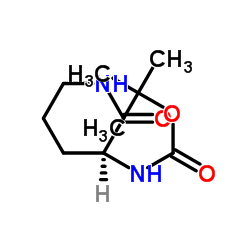

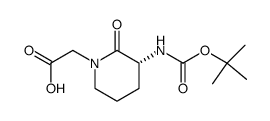

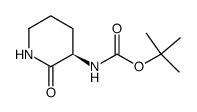
![benzyl 2-[(3S)-3-[(2-methylpropan-2-yl)oxycarbonylamino]-2-oxopiperidin-1-yl]acetate Structure](https://image.chemsrc.com/caspic/079/175133-83-2.png)