|
~92% |
|
~99% |
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~%
Detail
|
|
~% |
|
~83% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
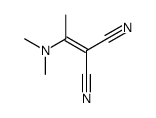
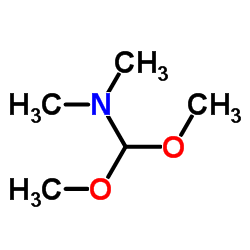
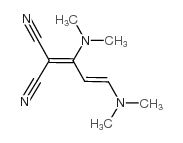
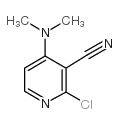
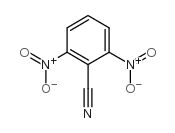


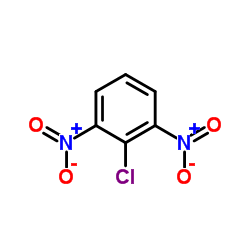
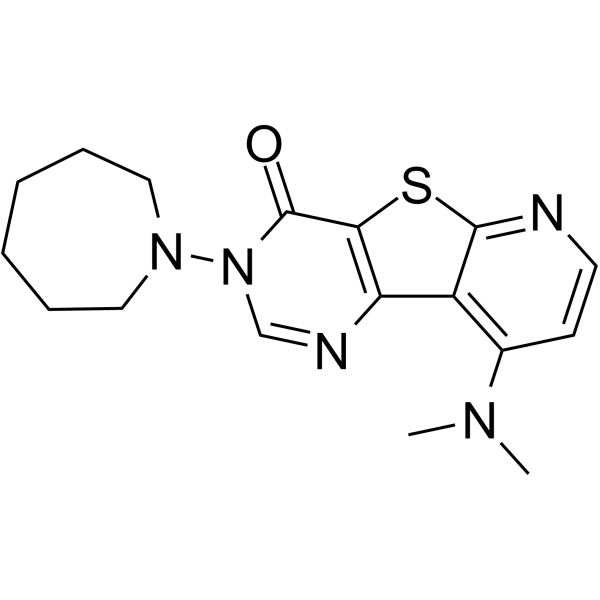
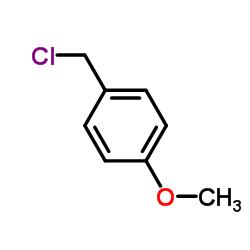
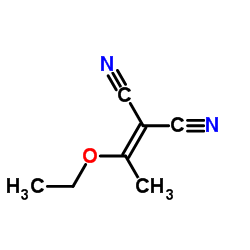
![2-bromo-4-[(4-methoxyphenyl)methoxy]pyridine-3-carbonitrile Structure](https://image.chemsrc.com/caspic/277/635731-99-6.png)
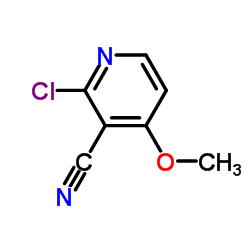
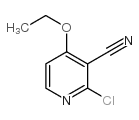
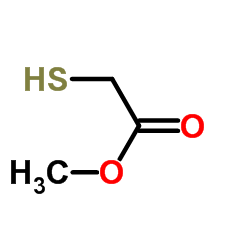
![3-AMINO-4-DIMETHYLAMINO-THIENO[2,3-B]PYRIDINE-2-CARBOXYLIC ACID METHYL ESTER Structure](https://image.chemsrc.com/caspic/239/331857-03-5.png)
