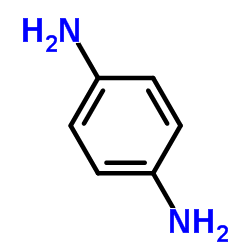
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
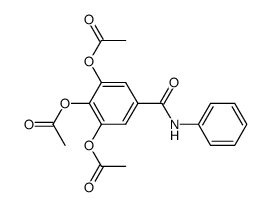
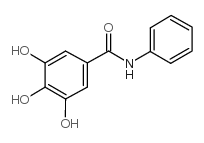

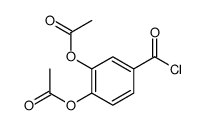
![N-[2-[(3,4-dihydroxybenzoyl)amino]phenyl]-3,4-dihydroxybenzamide Structure](https://image.chemsrc.com/caspic/487/194469-86-8.png)
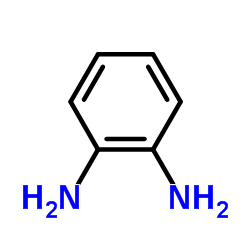
![N-[4-[(3,4-dihydroxybenzoyl)amino]phenyl]-3,4-dihydroxybenzamide Structure](https://image.chemsrc.com/caspic/004/353498-72-3.png)