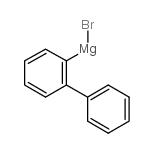
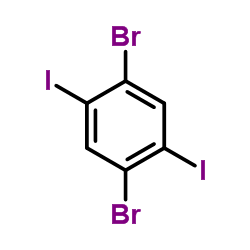
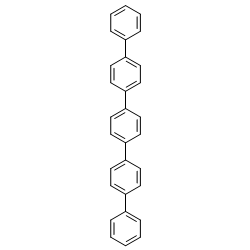
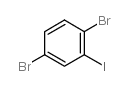
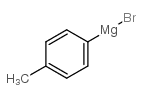
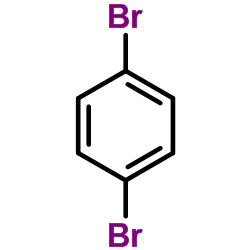
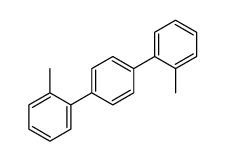
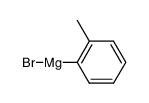
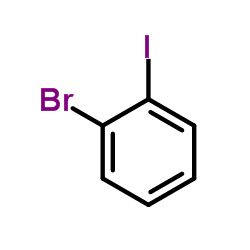
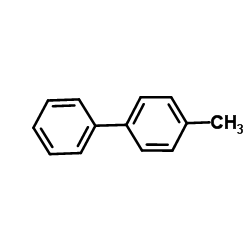
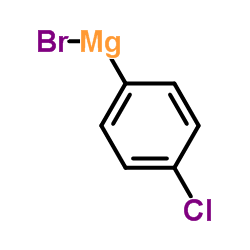
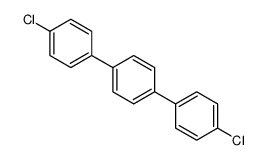
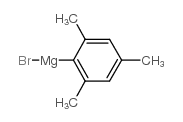
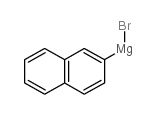
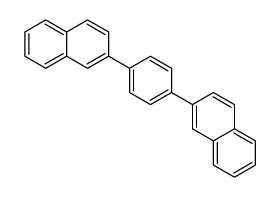
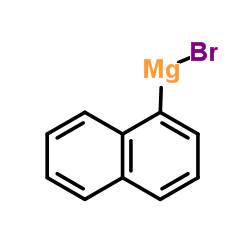
|
~0% |
|
~0% |
|
~% |
|
~% |
|
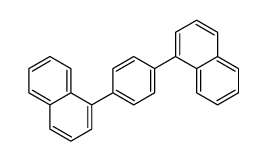
~71% |
|
~39% |
|
~43% |
|
~42% |
|
~39% |
|
~35% |
|
~17% |
|
~20% |
|
~% |
|
~0% |
|
~% |
|
~0% |