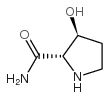
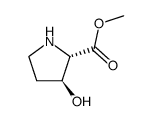
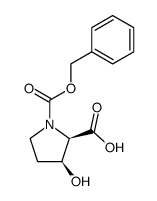
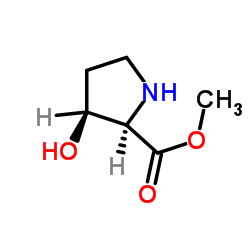
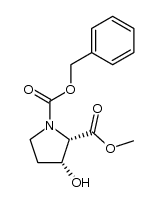
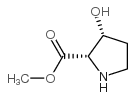
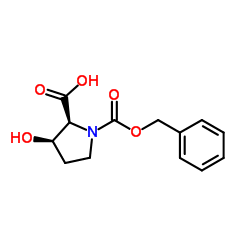
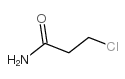
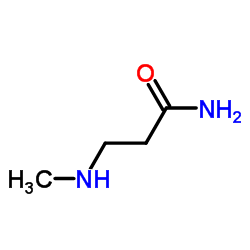
|
~% |
|
~% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~51% |
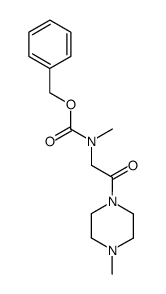
![Piperazine, 1-methyl-4-[(methylamino)acetyl]- (9CI) Structure](https://image.chemsrc.com/caspic/125/166187-00-4.png)

![(2S,3S)-1-[(benzyloxy)carbonyl]-3-hydroxypyrrolidine-2-carboxylic acid Structure](https://image.chemsrc.com/caspic/097/62182-54-1.png)