|
~76% |
|
~78% |
|
~79% |
|
~88% |
|
~77% |
|
~86% |
|
~14% |
|
~77% |
|
~87% |
|
~90% |
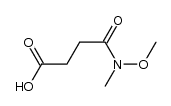
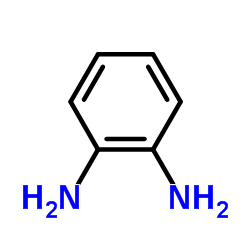
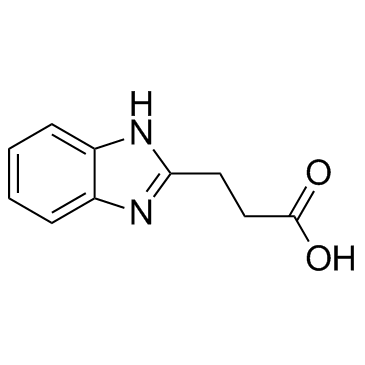
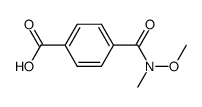
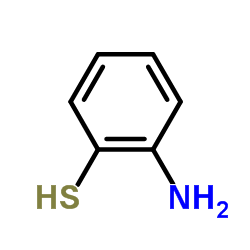
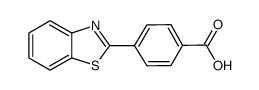
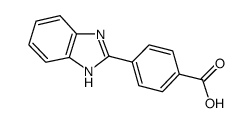
![3-(BENZO[D]THIAZOL-2-YL)PROPANOIC ACID Structure](https://image.chemsrc.com/caspic/354/29198-86-5.png)
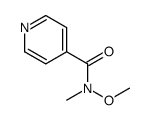
![2-(Pyridin-4-yl)benzo[d]thiazole Structure](https://image.chemsrc.com/caspic/216/2295-38-7.png)
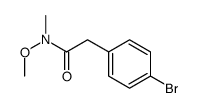
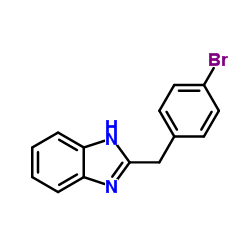

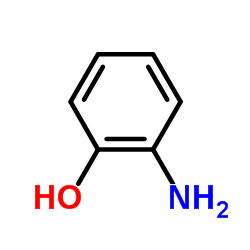
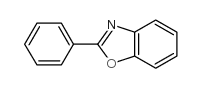

![2-Phenyl-1H-benzo[d]imidazole Structure](https://image.chemsrc.com/caspic/350/716-79-0.png)
![2-Methyl-1H-benzo[d]imidazole Structure](https://image.chemsrc.com/caspic/076/615-15-6.png)