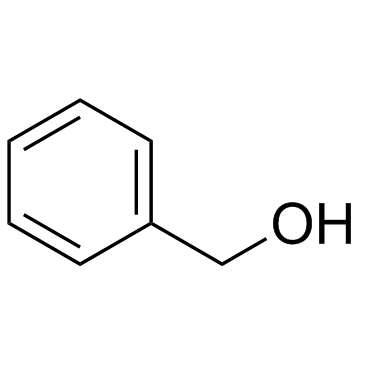
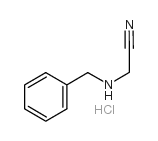
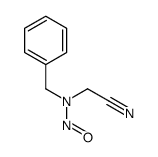
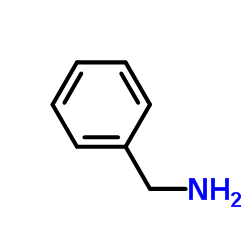
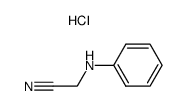
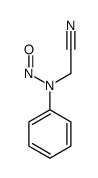
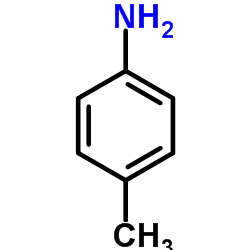
|
~% |
|
~46% |
|
~% |
|
~64% |
|
~30% |
|
~% |
|
~% |
![[N-Nitroso-N-benzyl-amino]-phenylacetonitril Structure](https://image.chemsrc.com/caspic/374/65551-49-7.png)

![7,14-diethyl-3,10-dimethyl-10,14-dihydrobenzo[1,2-g:4,5-g']dipteridine-2,4,9,11(1H,3H,7H,8H)-tetraone Structure](https://image.chemsrc.com/caspic/126/132949-38-3.png)