|
~% |
|
~% |
|
~% |
|
~% |
|
~70% |
|
~95% |
|
~% |
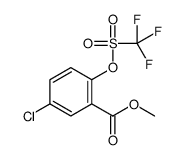
![tert-butyl N-[[2-(aminomethyl)-4-chlorophenyl]methyl]carbamate Structure](https://image.chemsrc.com/caspic/033/439116-15-1.png)


![[2-(aminomethyl)-5-chlorophenyl]methanol Structure](https://image.chemsrc.com/caspic/111/439117-39-2.png)
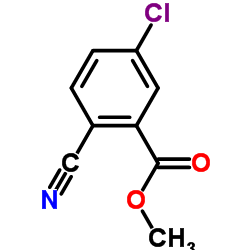
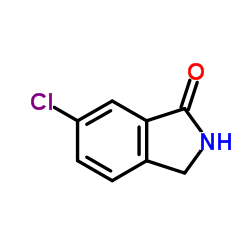

![tert-butyl N-[[4-chloro-2-(hydroxymethyl)phenyl]methyl]carbamate Structure](https://image.chemsrc.com/caspic/256/439117-40-5.png)