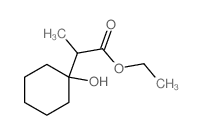
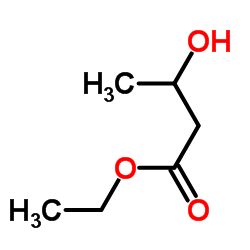
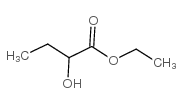
|
~24% |
|
~35% |
|
~75% |
|
~% |

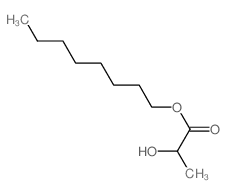
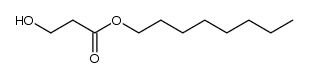
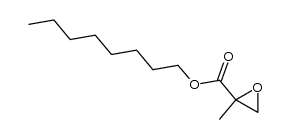
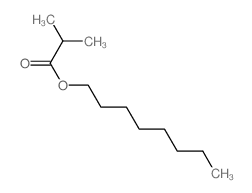
![ethyl 2-methyl-1-oxaspiro[2.5]octane-2-carboxylate Structure](https://image.chemsrc.com/caspic/325/31045-09-7.png)