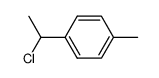
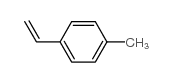
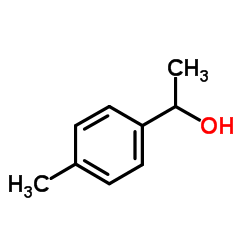
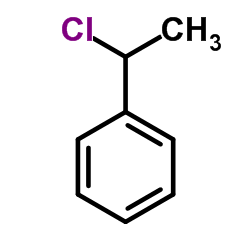
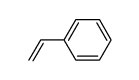
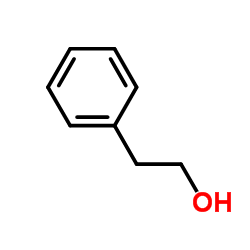
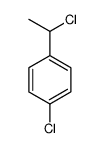
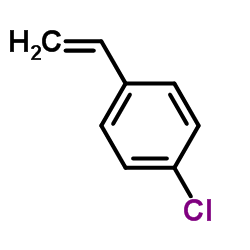
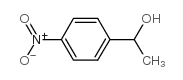
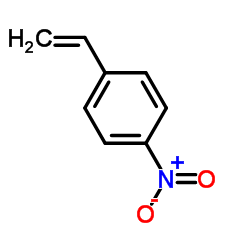
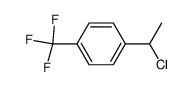
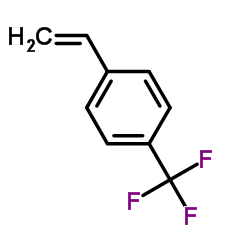
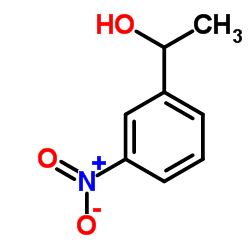
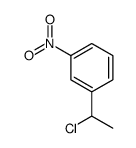
|
~7% |
|
~6% |
|
~51% |
|
~58% |
|
~% |
|
~0% |
|
~92% |