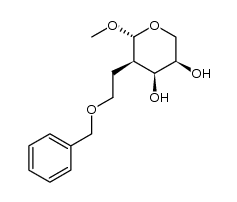
|
~% |
|
~% |
|
~82% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
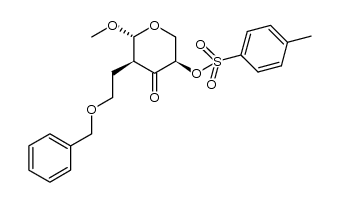

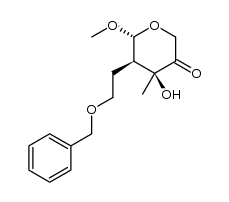
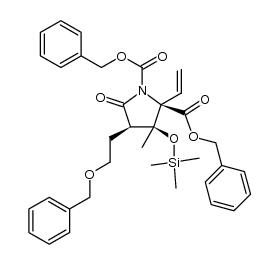
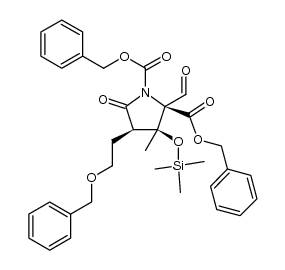
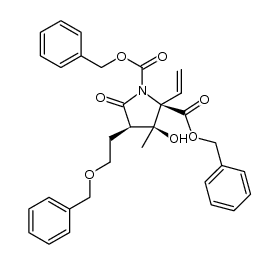

![2-((3aR,6R,7R,7aS)-6-methoxy-2,2-dimethyltetrahydro-3aH-[1,3]dioxolo[4,5-c]pyran-7-yl)ethanol Structure](https://image.chemsrc.com/caspic/336/1265173-01-0.png)