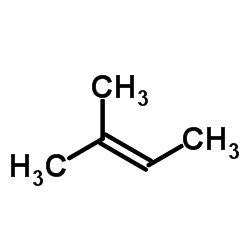
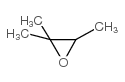
|
~41% |
|
~94% |
|
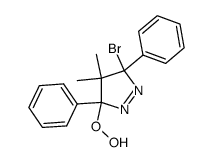
~85% |
|
~78% |
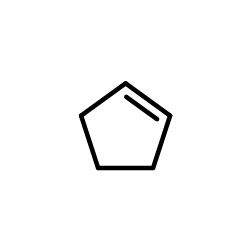

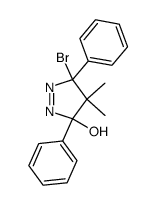
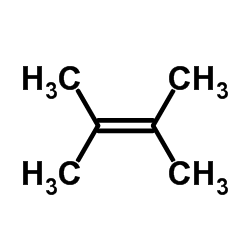
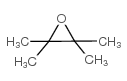
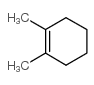
![1,6-dimethyl-7-oxabicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/351/17612-36-1.png)