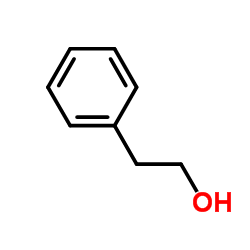
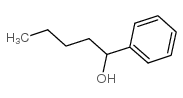
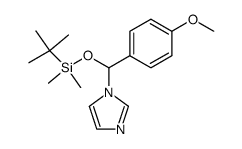
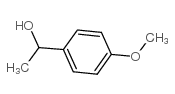
|
~81% |
|
~91% |
|
~87% |
|
~94% |
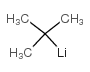

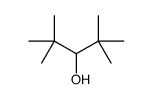

![tert-butyl-[imidazol-1-yl(phenyl)methoxy]-dimethylsilane Structure](https://image.chemsrc.com/caspic/151/410097-82-4.png)